PRECLINICAL CRO SERVICES FOR DRUGS TARGETING METABOLIC DISORDERS
Physiogenex provides preclinical in vivo and ex vivo CRO services with 20 years of expertise in evaluating drugs targeting metabolic disorders such as obesity, diabetes, NASH/MASH, fibrosis, diabetic nephropathy, cardiovascular complications, dyslipidemia and atherosclerosis.
We develop in-house and gold standard preclinical models in our cutting-edge facilities near Toulouse, France.
Along with our preclinical CRO services, we offer consulting services adapted to the specific needs of our clients. Our metabolic diseases experts offer their many years of experience in drug development to help you design and run efficient and cost-effective studies to shorten your product’s time to market.
STATE OF THE ART CRO FACILITIES
Physiogenex is located near Toulouse (France) in brand new facilities . Our technical platform is equipped with all appropriate devices to ensure high quality CRO services and reliable study results to our clients.
High success rate customer satisfaction in 2023: 97.5%
When is your turn?
THEY TRUST US
We work with more than 100 biopharmaceutical, nutraceutical and agrifood companies

LATEST NEWS ABOUT PHYSIOGENEX
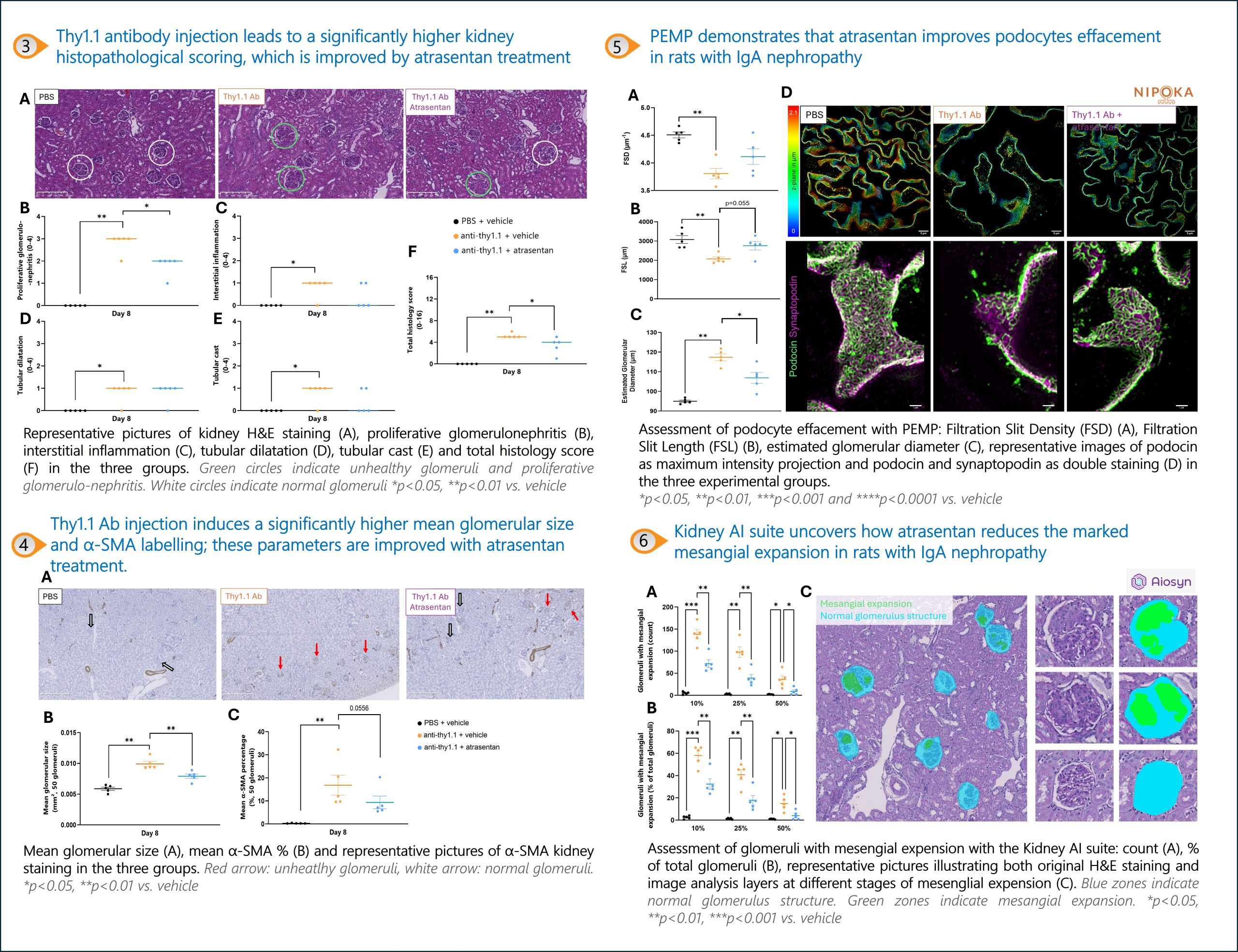
Physiogenex launches its new IgA nephropathy rat after presenting the model ASN Kidney Week
Physiogenex now delivers its novel rat model of IgA nephropathy (IgAN) to rapidly evaluate the efficacy of drugs targeting IgAN, in only 8 days! The model has…
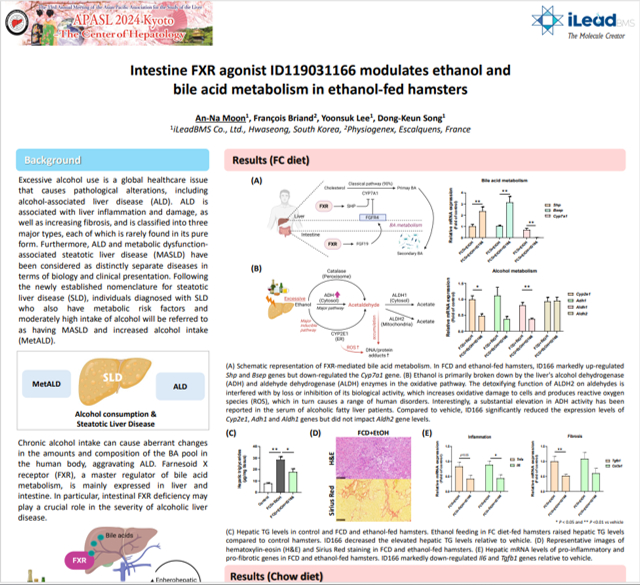
iLEADS BMS presents the effects of its novel FXR agonist in Physiogenex’s ALD/MetALD hamster models at APASL 2024, Kyoto, Japan
iLEADS BMS presents the effects of its novel FXR agonist in Physiogenex’s ALD/MetALD hamster models – check the poster content here If you are…
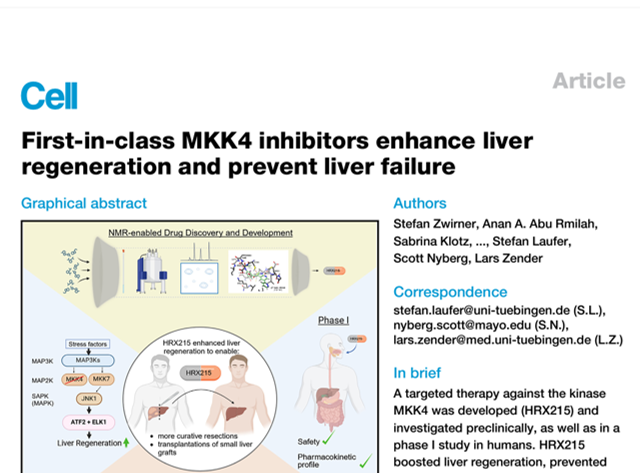
HepaRegenix publishes data on first in class MKK4 inhibitors demonstrating benefits in Physiogenex CCl4 mouse model of liver fibrosis
HepaRegenix publishes data on first in class MKK4 inhibitors demonstrating benefits in Physiogenex CCl4 mouse model of liver fibrosis. Review the manuscript…